Abstract
We assess repeatability of automatic measurements of a new anterior segment optical coherence tomographer and biometer (ANTERION) and their agreement with those provided by an anterior segment-optical coherence tomography device combined with Placido-disk corneal topography (MS-39) and a validated optical biometer (IOLMaster 500). A consecutive series of patients underwent three measurements with ANTERION and one with MS-39. A subgroup of patients underwent biometry also with IOLMaster 500. Repeatability was assessed by means of within-subject standard deviation, coefficient of variation (COV), and intraclass correlation coefficient (ICC). Agreement was investigated with the 95% limits of agreement. Paired t-test and Wilcoxon matched-pairs test were performed to compare the measurements of the different devices. Repeatability of ANTERION measurements was high, with ICC > 0.98 for all parameters except astigmatism (0.963); all parameters apart from those related to astigmatism revealed a COV < 1%. Repeatability of astigmatism improved when only eyes whose keratometric astigmatism was higher than 1.0 D were investigated. Most measurements by ANTERION and MS-39 showed good agreement. No significant differences were found between measurements by ANTERION and IOLMaster, but for corneal diameter. ANTERION revealed high repeatability of automatic measurements and good agreement with both MS-39 and IOLMaster for most parameters.
Similar content being viewed by others
Introduction
Imaging of the anterior segment has greatly improved in recent years thanks to the introduction of new technologies and the evolution of older ones. Such improvements enable us to obtain unprecedented qualitative information and quantitative measurements of the cornea, anterior chamber, irido-corneal angle, crystalline lens or intraocular lens (IOL).
Optical coherence tomography (OCT), which was initially developed for posterior segment imaging, is now being commonly used also for anterior segment analysis. The first generation of anterior segment optical coherence tomography (AS-OCT), which included instruments such as the Visante (Carl Zeiss Meditec, Germany), used a time-domain system at a central wavelength of 1310 nm but with low axial resolution (18- 25 μm) and a low scan speed (2000 A-scans/second)1,2. Those limits were overcome by introducing spectral-domain OCT (SD-OCT) technology, with a higher imaging speed and a better axial resolution3,4,5. Nevertheless, as they used a shorter wavelength light source (around 840 nm), SD-OCT devices had a limited image depth range. Recently, the use of a longer wavelength light source combined with swept-source OCT (SS-OCT) technology has allowed a greater image depth and high-contrast imaging of the entire anterior segment, from the cornea to the posterior surface of the lens6,7.
The ANTERION (Heidelberg Engineering, Germany) is a new high-resolution AS-OCT that combines a long wavelength light source with SS-OCT technology8. It provides OCT images and information detailing images of the structures from the anterior corneal surface to the posterior lens surface. Moreover, its technology makes it possible to obtain complete biometric measurements of the eye, so that intraocular lens (IOL) power can be calculated.
As with any newly introduced instrument, precision and accuracy are two mandatory requirements. The purpose of this study was to assess the repeatability of automatic measurements of the ANTERION in healthy patients and to assess their agreement with the corresponding parameters provided by an AS-OCT device combined with Placido-disk corneal topography (MS-39, CSO, Florence, Italy) and a validated optical biometer, the IOLMaster 500 (Carl Zeiss Meditec, Germany).
Results
Ninety-six eyes of 96 patients (mean age = 69.1 ± 16.7 years (range: 19—91) years; females = 58, 60.4%) were enrolled in the repeatability study. Table 1 shows that the repeatability of the ANTERION measurements was high, with ICC > 0.98 for all parameters except astigmatism (0.963) and all parameters apart from those related to astigmatism revealed a COV < 1%.
Astigmatism measurements (magnitude and axis of both keratometric and TCP astigmatism) showed a slightly worse repeatability compared to the other parameters. The repeatability significantly improved when only eyes whose keratometric astigmatism was higher than 1.0 D in all 3 measurements (n = 25, Table 2) were investigated, as the COV decreased below 7%. When the whole sample and the subgroup with keratometric astigmatism > 1.0 D were compared, the F-test confirmed that the difference in variance was statistically significant for the keratometric astigmatism magnitude (variance ratio = 7.31, p < 0.001), keratometric astigmatism axis (variance ratio = 28,428.31, p < 0.001), TCP astigmatism magnitude (variance ratio = 21.18, p < 0.001) and TCP astigmatism axis (variance ratio = 1976.24, p < 0.001).
Table 3 shows the comparison between the measurements of the ANTERION (first scan only) and MS-39. With both devices the mean simulated keratometry was higher than the mean TCP (p < 0.0001). Two statistically significant differences were found between the two devices as regards: 1) the posterior keratometry, which was higher (i.e. closer to zero) with the ANTERION, and 2) the magnitude of the TCP astigmatism, which was higher with the MS-39. Low agreement was found for the TCP astigmatism magnitude and axis, as well as for the corneal diameter. The remaining parameters showed good to excellent agreement.
A subgroup of fifty-seven eyes of 57 patients scheduled for cataract surgery (mean age = 75.2 ± 6.1 years (range: 60—88 years); females = 36, 63.2%) underwent optical biometry with both the ANTERION and IOLMaster 500, which showed a high rate of measurement success (55 eyes out of 57, 96.5%, with each device). Overall, the examination was successful with both instruments in 53 eyes (mean age = 75.5 ± 6.1 years, range: 60 – 88 years; females = 34, 64.2%). Table 4 reports the mean values and their agreement.
Discussion
The application of SS-OCT technology to anterior segment tomography and biometry has reduced the time of image acquisition and improved axial and lateral resolution and tissue penetration8,9. The aim of our study was to investigate the measurements provided by one of the latest SS-OCT optical biometers, the ANTERION, in order to assess its repeatability as well as its agreement with an AS-OCT and with a validated traditional optical biometer.
The precision of anterior segment measurements is crucial when planning refractive or cataract surgery and in order to evaluate the progression of corneal diseases, such as keratoconus.
Our data show a high repeatability of the measurements obtained with the ANTERION, as the values showed an ICC > 0.9. Our results about repeatability are in line with those recently published10. As previously reported for other devices11,12,13, the repeatability was slightly lower for astigmatism magnitude and axis. However, if we consider the subgroup including only eyes with a keratometric astigmatism higher than 1 D, the repeatability improved considerably. This is important, because keratometric astigmatism measurement is used to calculate the cylinder of toric IOLs, which are usually implanted in eyes with astigmatism higher than 1 D.
In our study we also compared the agreement between ANTERION measurements and the corresponding parameters measured with the MS-39, which has already been found to achieve high repeatability3,4,5,6,7,8,9,10,11,12,13,14. Most measurements (Sim-K, TCP, CCT, CD and AQD) revealed good agreement without statistically significant differences. However, a statistically significant difference and a lower agreement were found for posterior keratometry and TCP astigmatism magnitude measurements. While the difference in the case of the former parameter seems clinically negligible, the latter may have an impact for toric intraocular lens (IOL) calculations and therefore deserves future studies in this context. Differences between tomographer measurements of total corneal astigmatism have already been reported and may be related to the different technologies they are based on15.
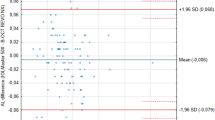
The comparison with the IOLMaster also revealed good agreement for all parameters. Measurements of AL were significantly different from a statistical, but not clinical point of view, with a mean discrepancy of just 0.01 mm, and agreement was high. These results, which are consistent with those previously reported in a study comparing the IOLMaster with the LenStar LS900 (Haag-Streit)16, may be explained by the fact that both the ANTERION and the IOLMaster use the group refractive index to convert the optical path length into a physical AL, as recommended by Haigis in 19999. This is the reason why agreement for AL measurements by different optical biometers has always been reported to be high7,17,18,19.
In our study, measurement of AL using the ANTERION had a high rate of success (96.5%), in line with previous studies that have described a range from 92 to 100% for different SS-OCT devices20,21,22 In contrast with the literature, which has reported a lower success rate in AL measurement using the IOLMaster 50021,23, our data showed the same success rate for the ANTERION and IOLMaster 500. As an explanation, the authors suggest the fact that the group of patients in this study did not include opaque cataracts.
As regards ACD measurements, our data must be interpreted with caution, since the current version of ANTERION software does not automatically supply this parameter, which we calculated by adding the measured AQD to CCT. Previous studies have found that the IOLMaster 500, which measures ACD through a lateral source of light, usually provides lower mean values compared to other optical biometers16,24,25,26,27,28,29,30,31. Future studies will need to assess whether ACD measurements provided automatically by the ANTERION (when commercially available) are close to those of other optical biometers.
Some limitations of our study should be considered. We excluded pathological eyes and postoperatively altered corneas, which deserve further investigations. Moreover, we did not assess the accuracy of ANTERION measurements for IOL power calculation; however, a study on this subject is already under way.
In conclusion, based on the findings of our study, the measurements provided by the new SS-OCT ANTERION show high repeatability and good agreement with those of the MS-39 for anterior segment parameters and with the IOLMaster 500 for biometric parameters.
This means that most measurements by the ANTERION and MS-39 can be used interchangeably in clinical practice and that the formula constants optimized for the IOLMaster 500 are likely to be valid for the ANTERION too.
Materials and methods
Participants
In this prospective study, we included a consecutive series of patients examined at the Anterior Segment Unit of G.B. Bietti Foundation IRCCS, Rome, Italy between October 2019 and January 2020. Subjects aged ≥ 18 years and without a history of ocular surgery, contact lens wear in the last month, corneal disease or trauma were eligible for enrollment. All patients underwent a complete ophthalmic evaluation, including distance-corrected visual acuity measurement, anterior segment biomicroscopy, Goldmann applanation tonometry and fundus biomicroscopy. For the purposes of this study, anterior segment tomography and optical biometry were performed in all subjects using the ANTERION Cataract App. Anterior segment tomography was performed in all eyes with the MS-39. Finally, a subgroup of patients scheduled for cataract surgery underwent optical biometry also with the IOLMaster 500.
We excluded patients with ocular diseases that could affect fixation and the quality of acquisition, such as corneal opacity or any maculopathy. Other exclusion criteria were the presence of keratoconus or suspect keratoconus, as shown by corneal tomography with the MS-39, and contact lens use in the past month. All research procedures described in this work adhered to the tenets of the Declaration of Helsinki.
All recruited subjects gave written informed consent. The study protocol was approved by the Ethics Committee of G.B. Bietti Foundation.
Instruments
The ANTERION is a swept-source AS-OCT with a 1300 nm wavelength light source, with a speed of 50,000 A-scans/second. It images the anterior segment of the eye with an axial depth of 14 mm, a lateral width of 16.5 mm, an in tissue axial resolution of < 10 µm and lateral resolution of 30–45 mm8. All measurements are assisted by an eye-tracking technology, centered on the corneal vertex. Corneal information is based on 65 radial scans, generating data for corneal maps and analysis at 8 mm diameter. The Cataract App acquisition was used to acquire the data (software version 1.2) and the following parameters were analyzed:
-
Simulated keratometry (Sim-K) average: this is defined as the average corneal power calculated at a 3 mm ring, based on anterior corneal axial curvature. The conversion of anterior radii to keratometry values is performed using the standard keratometric index of 1.3375.
-
Keratometric astigmatism: this is defined as the dioptric difference between the steep and the flat corneal meridians, as calculated by Sim-K.
-
Posterior keratometry average: this is defined as the posterior corneal power calculated at a 3 mm ring, based on the posterior corneal axial curvature. The curvature of posterior radii to keratometry values is performed using the refractive indices of the cornea (1.376) and aqueous (1.336).
-
Total corneal power (TCP) average: this is defined as the average refractive power of the cornea, calculated by ray tracing at a 3 mm ring through both corneal surfaces. Ray tracing determines how parallel light beams are refracted according to the slope of the cornea, the true refractive indices (ncornea = 1.376, naqueous = 1.336), and the exact point of refraction.
-
TCP astigmatism: this is defined as the dioptric difference between the steep and the flat corneal meridians, as calculated on the basis of TCP.
-
Central corneal thickness (CCT): this is defined as the perpendicular distance between the central anterior and posterior corneal surfaces, measured from the anterior corneal vertex.
-
Corneal diameter (CD): this parameter, which is defined as white-to-white on the instrument, is measured as the horizontal distance between the nasal and temporal limbus, as obtained on the camera image.
-
Aqueous depth (AQD): this is defined as the distance from the central posterior corneal surface to the central anterior lens surface, measured perpendicular to the anterior corneal surface and along the line of sight. By adding AQD and CCT it is possible to calculate the anterior chamber depth (ACD) as the distance from the corneal epithelium to the anterior lens surface as used in IOL power formulas.
-
Lens thickness (LT): this is defined as the distance between the central anterior and posterior lens surfaces, measured perpendicular to the anterior corneal surface;
-
Axial length (AL): this is defined as the distance between the anterior corneal surface and the retinal pigment epithelium, along the line of sight. AL is calculated by means of a group refractive index.
The MS-39 (software version 3.6) is a spectral-domain OCT combined with a Placido disk. It acquires one Placido top-view image and a series of 25 SD-OCT radial scans at a wavelength of 840 nm, with an axial resolution of 3.5 µm in tissue, a lateral resolution of 35 µm and a depth of 7.5 mm. Data for the anterior surface from the Placido image and SD-OCT scans are merged using a proprietary method. High repeatability of its measurements has been previously reported3.
The IOLMaster 500 (software version 5.4) is a first generation optical biometer using partial coherence interferometry (PCI) for AL measurement. The keratometry value is measured by means of a six-point telecentric reflectometry technique in an approximately 2.5 mm diameter pattern, while ACD is measured by lateral slit beam illumination. Several articles have confirmed the high repeatability of its measurements32,33,34,35.
Measurement procedures
One eye of each patient was randomly selected. ANTERION and MS-39 scans were taken on the same day, between 11 A.M. and 5 P.M., to minimize diurnal changes. Examinations were performed without pupil dilation in a dimly lit room, after each instrument had been calibrated at the beginning of the day. In order to assess the repeatability of ANTERION measurements, the tomographic and biometric parameters were acquired 3 times by an experienced examiner (MR). After each measurement, the patient was invited to sit back and the device was realigned before the subsequent scan.
With the IOLMaster 500, the measurements were acquired only once if the scan quality, as indicated by the instrument, was good; otherwise, measurements were repeated until a good quality was achieved.
Statistical analysis
The repeatability analysis was carried out according to the standards of the International Organization for Standardization36. The following parameters were investigated:
-
1.
Pooled within-subject standard deviation (Sw) and intra-session test–retest variability (also known as repeatability or limits of repeatability). The latter was obtained by multiplying the Sw by 2.7737. Test–retest repeatability indicates an estimate of the limits within which 95% of measurements for the same subject should be.
-
2.
Coefficient of variation (COV). This was calculated as the Sw divided by the mean of the measurements and expressed as a percentage38.
-
3.
Intraclass correlation coefficient (ICC). This is the ratio of the between-subjects variance to the sum of the Sw and the between-subjects variance. The ICC, which is close to 1.0 when there is no variance between repeated measurements, was automatically calculated using the 2-way mixed model and absolute agreement. ICCs ranging from 0 to 1 are classified as follows: ICC less than 0.75 = poor agreement; ICC 0.75 to less than 0.90 = moderate agreement and ICC 0.90 and more = high agreement39.
The variance of different measurements was compared by means of the F-test.
Based on the paper by McAlinden40, a minimum sample size of 96 eyes was estimated to be necessary to assess repeatability: this sample size allowed us to have a 0.10 confidence in the estimate with 3 repeated measures.
Agreement among the measurements provided by the different devices was assessed by means of the 95% limits of agreement (LoA)41. The difference between measurements (y-axis) is plotted against their means (x-axis). The mean difference is the estimated bias and the standard deviation (SD) of the differences shows the fluctuation around the mean. The 95% LoAs are calculated as the mean ± 1.96 SD of the differences between the 2 measurement techniques. The sample size for the agreement between ANTERION biometry and IOLMaster was calculated, according the recommendation of McAlinden42 using the following approximated formula: 1.96 \(\frac{{\sqrt {3s}^{2} }}{\surd n} =\) desired confidence of LoA.
We speculated that the desired confidence of LoA for axial length was 0.02 with a standard deviation of mean difference (s) 0.04, which was not clinically important.
We also calculated the ICC to complete our analysis of agreement between the instruments (ANTERION versus MS-39 and versus IOLMaster 500, respectively).
In addition, Statistics were analyzed by means of MedCalc (version 12.3.0.0, MedCalc Software Ltd, Ostend, Belgium) and Instat (version 3.10, Graphpad Software Inc, La Jolla, CA).
Data availability
The datasets used and analysed to support the findings of this study are available from the corresponding author upon request.
References
Mohamed, S. et al. Repeatability and reproducibility of pachymetric mapping with Visante anterior segment—optical coherence tomography. Invest. Ophthalmol. Vis. Sci. 48, 5499–5504 (2007).
Ang, M. et al. Anterior segment optical coherence tomography. Prog. Retin. Eye Res. 66, 132–156 (2018).
Savini, G., Schiano-Lomoriello, D. & Hoffer, K. J. Repeatability of automatic measurements by a new anterior segment optical coherence tomographer combined with Placido topography and agreement with 2 Scheimpflug cameras. J Cataract Refract Surg 44, 471–478 (2018).
Tang, M., Chen, A., Li, Y. & Huang, D. Corneal power measurement with Fourier-domain optical coherence tomography. J. Cataract. Refract. Surg. 36, 2115–2122 (2010).
Mansoori, T. & Balakrishna, N. Intrasession repeatability of pachymetry measurements with RTVue XR 100 optical coherence tomography in normal cornea. Saudi J. Ophthalmol. 31, 65–68 (2017).
Shoji, T. et al. In vivo crystalline lens measurements with novel swept-source optical coherent tomography: an investigation on variability of measurement. BMJ Open Ophthalmol. 1, e000058 (2017).
Fukuda, S. et al. Comparison of anterior segment and lens biometric measurements in patients with cataract. Graefes Arch. Clin. Exp. Ophthalmol. 258, 137–146 (2020).
Asam, J.S., Polzer, M., Tafreshi, A., Hirnschall, N. & Findl, O. Anterior segment OCT. In: Bille JF, Ed.: High resolution imaging in microscopy and ophthalmology: new frontiers in biomedical optics. Cham (CH). Chapter 13 pp. 285–299 (Springer, 2019).
Wojtkowski, M., Kaluzny, B. & Zawadzki, R. J. New directions in ophthalmic optical coherence tomography. Optom. Vis. Sci. 89, 524–542 (2012).
Ruíz-Mesa, R. et al. Ocular biometric repeatability using a new high-resolution swept-source optical coherence tomographer. Expert Rev. Med. Devices 17, 591–597 (2020).
Ventura, B. V., Al-Mohtaseb, Z., Wang, L., Koch, D. D. & Weikert, M. P. Repeatability and comparability of corneal power and corneal astigmatism obtained from a point-source color light-emitting diode topographer, a Placido-based corneal topographer, and a low-coherence reflectometer. J. Cataract. Refract. Surg. 41, 2242–2250 (2015).
Kim, E. J. et al. Repeatability of posterior and total corneal curvature measurements with a dual Scheimpflug-Placido tomographer. J. Cataract. Refract. Surg. 41, 2731–2738 (2015).
Aramberri, J. et al. Dual versus single Scheimpflug camera for anterior segment analysis: precision and agreement. J. Cataract. Refract. Surg. 38, 1934–1949 (2012).
Savini, G., Schiano-Lomoriello, D. & Hoffer, K. J. Repeatability of automatic measurements by a new anterior segment optical coherence tomographer combined with Placido topography and agreement with 2 Scheimpflug cameras. J. Cataract. Refract. Surg. 4, 471–478 (2018).
Savini, G., Næser, K., Schiano-Lomoriello, D. & Ducoli, P. Total corneal astigmatism measurements: agreement between 2 rotating Scheimpflug cameras. Cornea 36, 463–469 (2017).
Hoffer, K. J., Shammas, H. J. & Savini, G. Comparison of 2 laser instruments for measuring axial length. J. Cataract. Refract. Surg. 36, 644–648 (2010).
Srivannaboon, S., Chirapapaisan, C., Chonpimai, P. & Loket, S. Clinical comparison of a new swept-source optical coherence tomography-based optical biometer and a time-domain optical coherence tomography-based optical biometer. J. Cataract. Refract. Surg. 41, 2224–2232 (2015).
Ferrer-Blasco, T. et al. Evaluation of the repeatability of a swept-source ocular biometer for measuring ocular biometric parameters. Graefes Arch. Clin. Exp. Ophthalmol. 255, 343–349 (2017).
Yeu, E. Agreement of ocular biometry measurements between 2 biometers. J. Cataract. Refract. Surg. 45, 1130–1134 (2019).
Akman, A., Asena, L. & Güngör, S. G. Evaluation and comparison of the new swept source OCT-based IOLMaster 700 with the IOLMaster 500. Br. J. Ophthalmol. 100, 1201–1205 (2016).
Shammas, H. J., Ortiz, S., Shammas, M. C., Kim, S. H. & Chong, C. Biometry measurements using a new large-coherence-length swept-source optical coherence tomographer. J. Cataract. Refract. Surg. 42, 50–61 (2016).
Kurian, M. et al. Biometry with a new swept-source optical coherence tomography biometer: repeatability and agreement with an optical low-coherence reflectometry device. J. Cataract. Refract. Surg. 42, 577–581 (2016).
Sabatino, F., Matarazzo, F., Findl, O. & Maurino, V. Comparative Analysis of 2 Swept-Source Optical Coherence Tomography Biometers. J. Cataract. Refract. Surg. 45, 1124–1129 (2019).
Holzer, M. P., Mamusa, M. & Auffarth, G. U. Accuracy of a new partial coherence interferometry analyzer for biometric measurements. Br. J. Ophthalmol. 93, 807–810 (2009).
Buckhurst, P. J. et al. A new optical low coherence reflectometry device for ocular biometry in cataract patients. Br. J. Ophthalmol. 93, 943–953 (2009).
Rabsilber, T. M., Jepsen, C., Auffarth, G. U. & Holzer, M. P. Intraocular lens power calculation: clinical comparison of 2 optical biometry devices. J. Cataract. Refract. Surg. 36, 230–234 (2010).
Hoffer, K. J., Hoffmann, P. C. & Savini, G. Comparison of a new optical biometer using swept-source optical coherence tomography and a biometer using optical low-coherence reflectometry. J. Cataract. Refract. Surg. 42, 1165–1172 (2016).
Hoffer, K. J., Shammas, H. J., Savini, G. & Huang, J. Multicenter study of optical low-coherence interferometry and partial-coherence interferometry optical biometers with patients from the United States and China. J. Cataract. Refract. Surg. 42, 62–67 (2016).
Huang, J. et al. Repeatability and interobserver reproducibility of a new optical biometer based on swept-source optical coherence tomography and comparison with IOLMaster. Br. J. Ophthalmol. 101, 493–498 (2017).
Savini, G. et al. Accuracy of a new swept-source optical coherence tomography biometer for IOL power calculation and comparison to IOLMaster. J. Refract. Surg. 33, 690–695 (2017).
Hoffer, K. J. & Savini, G. Comparison of AL-Scan and IOLMaster 500 partial coherence interferometry optical biometers. J. Refract. Surg. 32, 694–698 (2016).
Haigis, W., Lege, B., Miller, N. & Schneider, B. Comparison of immersion ultrasound biometry and partial coherence interferometry for intraocular lens calculation according to Haigis. Graefes Arch. Clin. Exp. Ophthalmol. 238, 765–773 (2000).
Vogel, A., Dick, H. B. & Krummenauer, F. Reproducibility of optical biometry using partial coherence interferometry : intraobserver and interobserver reliability. J. Cataract. Refract. Surg. 27, 1961–1968 (2001).
Olsen, T. Improved accuracy of intraocular lens power calculation with the Zeiss IOLMaster. Acta Ophthalmol Scand. 85, 84–87 (2006).
Chen, Y. A., Hirnschall, N. & Findl, O. Evaluation of 2 new optical biometry devices and comparison with the current gold standard biometer. J Cataract Refract Surg. 37, 513–517 (2011).
International Organization for Standardization. Accuracy (Trueness and Precision) of measurement methods and results. Part 1. General principles and definitions. Geneva, Switzerland, ISO, 1994; (ISO 5725–1:1994).
Bland, J. M. & Altman, D. G. Measurement error [Statistics notes]. BMJ 313, 744 (1996).
Budenz, D. L., Fredette, M. J., Feuer, W. J. & Anderson, D. R. Reproducibility of peripapillary retinal nerve fiber thickness measurements with stratus OCT in glaucomatous eyes. Ophthalmol. 115, 661–666 (2008).
McGraw, K. O. & Wong, S. P. Forming inferences about some intraclass correlation coefficients. Psychol Meth. 1, 30–46 (1996).
McAlinden, C., Khadka, J. & Pesudovs, K. Precision (repeatability and reproducibility) studies and sample-size calculation [guest editorial]. J Cataract Refract Surg. 41, 2598–2604 (2015).
Bland, J. M. & Altman, D. G. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1, 307–310 (1986).
McAlinden, C., Khadka, J. & Pesudovs, K. Statistical methods for conducting agreement (comparison of clinical tests) and precision (repeatability or reproducibility) studies in optometry and ophthalmology Ophthalmic Physiol Opt 31, 330–338 (2011).
Acknowledgements
The authors thank Francesca Bongiorno and Marianna Rosati for their technical assistance. The contribution of IRCCS- Fondazione Bietti was supported by the Italian Ministry of Health and Fondazione Roma.
Funding
No funding or sponsorship was received for the publication of this article.
Author information
Authors and Affiliations
Contributions
Designed the study: D.S.L., K.J.H. and G.S.; Conducted the study: D.S.L. and I.A.; Collected the subjects: D.S.L. and I.A. Acquired the data: D.S.L. and I.A.; Analyzed the data and drafted the main manuscript: K.J.H., G.S. and I.A. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
Dr. Hoffer licenses the registered trademark name Hoffer to ensure accurate programming of his formulas to Carl Zeiss-Meditec (IOLMasters), Haag-Streit (LenStar/EyeStar), Heidelberg Engineering (ANTERION), Oculus (Pentacam AXL), Movu (Argos), Nidek (AL-Scan), Oculus (Pentacam AXL), Tomey (OA-2000), Topcon EU/VISIA Imaging (Aladdin), Ziemer (Galilei G6) and all A-scan biometer manufacturers. Dr. Savini is a consultant to CSO and has received personal fees from Alcon, Oculus and Zeiss. The remaining authors have no financial disclosures.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Schiano-Lomoriello, D., Hoffer, K.J., Abicca, I. et al. Repeatability of automated measurements by a new anterior segment optical coherence tomographer and biometer and agreement with standard devices. Sci Rep 11, 983 (2021). https://doi.org/10.1038/s41598-020-79674-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-79674-4
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.